“How long will this take to freeze or thaw?” This is a question that is regularly asked, and the short answer is always, “It depends!” I thought I would take an opportunity to build on Kristine’s fantastic article from August and highlight some of the history and science behind solid-liquid transitions as they occur in everyday foods and the complexity of why freezing and thawing can be so challenging.
Freezing is a method of preservation that we have record of using as far back as 1100 BCE. Egyptians were chronicled to have used evaporative cooling to create ice used to maintain the quality of food during feasts (1). We have long understood the positive impact that reduction of heat energy has on the quality of our fresh and prepared foods. When we freeze or cool a product, we remove heat energy which in turn slows down chemical reactions and stabilizes the current state of fruits and vegetables. This works to reduce the negative effects of spoilage organisms, enzymatic degradation, natural separations, and to preserve beneficial vitamins and minerals.
Just as there are a myriad of food combinations that we could freeze, there are many factors that come into play when we transition between a liquid state to a solid one. The first thing we must consider are the mechanics of heat transfer. There are three distinct types of heat transfer that have been classified based on the interactions required to transfer energy. Of these three, the two most heavily relied upon in the food industry are convection and conduction. Radiation will not be covered in depth here.
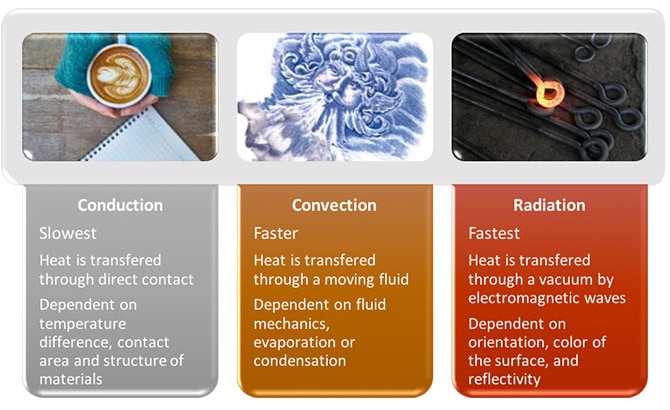
Figure 1: Comparison of heat transfer modes
Let’s consider the role of conduction. A product within a package needs to transfer heat in or out to thaw or freeze respectively. When we start looking at potential cooling or heating methods, consideration needs to be given to the container in which the product is held. Is it made from a material that can easily conduct heat like metal? Or is it insulated in a poor conductor like plastic or cardboard that does not allow easy passage of heat energy? You want to have a slightly higher thermal conductivity (see Fig 2) in your container than the contents within for effective cooling or thawing. Secondly, the thickness of the packaging material should be gauged. Thinner is better for heat transfer. To provide the swiftest cooling or heating to a product within a container, it is important that the package be a good conductor of heat energy and made of a thin sheet.
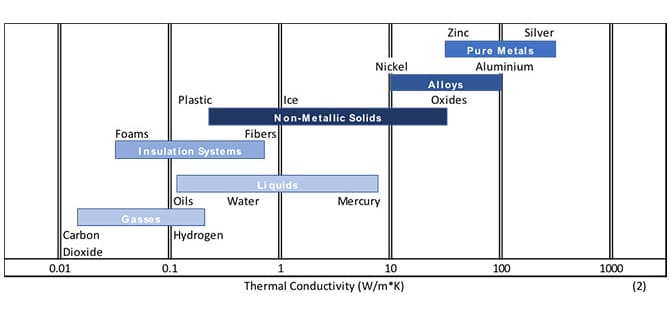
Figure 2: Relative conductive properties of various materials at normal pressures and temperatures (2)
Now, let’s focus on convection, the faster of the two heating methods. With convection a moving fluid, air or water, transfers heat into or out of a solid surface, our packaged product. The temperature difference between solid and fluid, fluid speed across the surface and the surface area will all impact the rate at which heat can be added to or removed from a product.
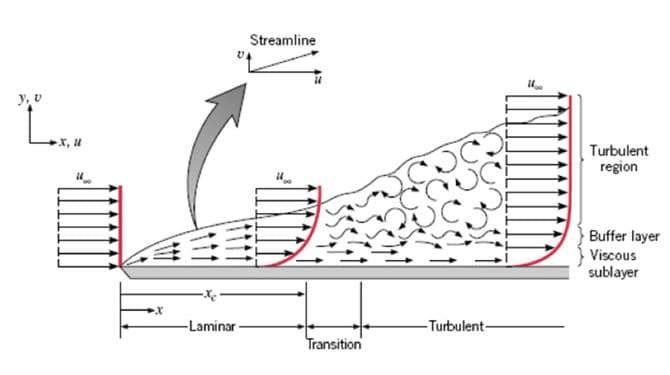
Figure 3: Velocity Boundary Layer Development (2)
As fluid moves past the surface, a phenomenon known as a boundary layer develops. This is where the fluid just at the very surface of the solid is not moving relative to the rest of the fluid. If we move out away from the solid surface by just a tiny bit, we see that the fluid does begin to move. Again, moving even farther away, the fluid will move faster, until you reach what is known as the freestream condition where more distance from the surface will no longer produce an increase in fluid velocity. Energy must pass from the non-moving surface fluid layer and through the adjacent layers to be removed from the surface. To enhance this energy transfer look to increase the speed of the fluid to a point where turbulent flows begin to be established. Turbulent flows are characterized by random and unpredictable interactions between particles of the fluid. Increasing fluid velocities to turbulent speeds will yield an increase in heat transfer rate.
How do we apply these ideas of heat transfer to our application? Let’s consider packing density in our heat transfer system, hypothetically a thawing area that has just been loaded with frozen product. Are packages stacked in such a way to allow air to flow freely around them? Or are the packages closely stacked and stretch wrapped, restricting heat transfer into the centrally placed products? Are we trying to thaw full or partial pallets? What about pallet-to-pallet interactions? Are rows of pallets blocking the ones behind from receiving heat at similar conditions to the front row? Understanding the impact of load configuration and fluid dynamics within your freezing or thawing systems can mean the difference between 4-hour and 8-hour treatments.
The specific formulation of a product plays a role in freeze/thaw characteristics as well. When you have pure water, it will freeze at 32 degrees Fahrenheit. When water freezes under normal atmospheric conditions, adjacent molecules form hydrogen bonds and order themselves into a ring structure. This solid structure has lower energy than liquid water. When you have a solution of mostly water with some sugar dissolved in it, you will find the freezing point of the solution to be lower than pure water. Sugar dissolves readily in water and does not want to get out of the way to allow those hydrogen bonds to form. This makes it harder for water to freeze. The new lower freezing temperature will directly relate to the amount of sugar in the water. From the graph below, you can see the influence that dissolved solids concentration has on freezing temperatures. This also explains how in a common freezer a lower brix solution will freeze sooner than one at higher brix.
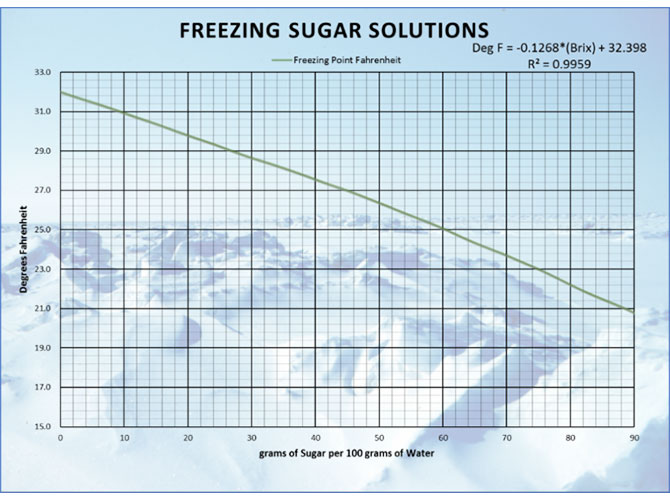
Figure 4: Correlation between sugar concentration and freezing point depression (3)
So how can we know how long something will take to freeze or thaw? While none of the information presented directly answers this question, it should highlight the complexity of freeze/thaw systems and some of the interactions that play major roles in how long it will take to reach a desired state. The easiest way to determine a freeze time is to test it in a real-world scenario. Thermal data loggers can be put into product at various locations and set to record the temperature at given time intervals. Product can then be frozen with these active data loggers and then thawed to yield a good idea of how long it will take to freeze or thaw a product under a given set of circumstances. Keep in mind, if circumstances of your system change – air velocity or temperatures, temperature of incoming product, pallet stacking patterns, package material thickness, and formulation – expect an impact to the answer.
References –
- Enochian, Robert V. and Willis R Woolrich. Fundamentals of Food Freezing. Westport: AVI Publishing Company Inc, 1977.
- Incropera, Frank P. and David DeWitt. Fundamentals of Heat and Mass Transfer. New York: John Wiley & Sons, 2002.
- Leighton, Alan. On the Calculation of the Freezing Point of Ice Cream Mixes and the Quantities of Ice Separated During the Freezing Process. Washington D.C.: Research Laboratories USDA, 1927.
For more information about Tree Top’s fruit ingredients, explore our product pages. Our research and development team is ready to assist you in finding the perfect fruit solution for your next formulation. Call us at (800) 367-6571 ext. 1435




